Nutryelt
A balanced parenteral nutrition (PN) multi-trace element solution containing nine essential trace elements that helps avoid micronutrient deficiencies and aligns with international guidelines.
When you give them macros, don’t forget the micros. Micronutrients are essential for the body to carry out and maintain multiple metabolic processes. Especially, in patients receiving PN, routine and appropriate provision of certain trace elements is necessary, not only to allow the patient’s underlying disease process to improve, but also to avoid deficiency or toxicity.1 Nutryelt is formulated to meet patients’ requirements of nine trace elements: selenium, zinc, copper, iron, manganese, fluoride, molybdenum, chromium, and iodine.2,3 Nutryelt can be used with macronutrients as part of the overall nutrition therapy of adult patients.3
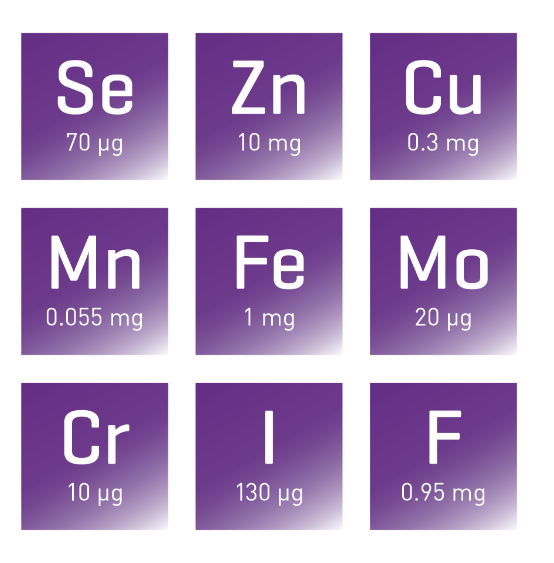
Nutryelt is aligned with international guidelines7,11-14
International guidelines recommend that trace elements should be provided daily to adult patients receiving PN therapy.1,2,6,7 Nutryelt provides a balanced concentrate of nine essential trace elements for adult patients receiving IV nutrition regimen. Nutryelt contains selenium, zinc, copper, iron, manganese, fluoride, molybdenum, chromium, and iodine, all of which are necessary to maintain multiple metabolic processes of the body.3
Formulated with a high zinc content
Nutryelt is formulated with a zinc content that helps meet the needs of patients with basal to moderately increased zinc requirements.2,7,8,11
Minimizes risk of toxicity
Reduced content of copper and manganese minimizes the risk of toxicity and excessive organ accumulation in patients on long-term PN therapy.1,9,10
Designed for ease of use
Polypropylene Luer-fit ampoule is associated with a low risk of breakages and allows needle-free connectivity, which eliminates the risk of needle stick injuries.15

No refrigeration required
Can be stored at room temperature for up to three years when unopened for enhanced convenience and access at point of care.3
Nutryelt
Download the Baxter Parenteral Nutrition Compatibility Guide for compounding and multi-chamber bag compatibility and stability information.
Learn more about Clinical Nutrition

Related Products
Important safety information
This abbreviated summary of product characteristics (SPC) is intended for international use. Please note that it may differ from the licensed SPC in the country where you are practicing. Therefore, please always consult your country-specific SPC or package leaflet.
NUTRYELT, concentrate for solution for infusion
QUALITATIVE AND QUANTITATIVE COMPOSITION
One ampoule (10 ml) contains: Zinc 10 mg; Copper 300 ug, Manganese 55 ug, Fluoride 950 ug, Iodine 130 ug, Selenium 70 ug, Molybdenum 20 ug, Chromium 10 ug, Iron 1 mg. Excipients: hydrochloric acid, water for injections.
CLINICAL PARTICULARS
Therapeutic indications:
NUTRYELT is used as part of an intravenous nutrition regimen, to cover basal or moderately increased trace element requirements in parenteral nutrition.
Posology and method of administration:
For adults only.
The recommended daily dose in patients with basal to moderately increased requirements is one ampoule (10 ml) of NUTRYELT. In cases of significantly increased trace element requirements (such as extensive burns, patients in severe hypercatabolic state due to major trauma) 2 ampoules (20 ml) of NUTRYELT may be given per day, and monitoring of serum trace element level is recommended.
Method of administration
NUTRYELT is not intended to be administered in its current presentation. It should be diluted according to the final desired osmolarity. The osmolarity value of the final preparation allows either administration through a peripheral vein, or only central venous catheter administration
Contraindications:
NUTRYELT must not be used in:
Children, patients less than 40 kg body weight, pronounced cholestasis (serum bilirubin > 140 μmol/l), hypersensitivity to the active substances and to the excipient, in cases of Wilson’s disease and hemochromatosis, if serum concentrations of any of the trace elements contained in NUTRYELT are elevated.
Special warnings and precautions for use:
The solution should be used after an accurate control of the patient clinical and biological parameters. Blood manganese levels should be regularly monitored in case of prolonged artificial nutrition: dose reduction may be necessary or NUTRYELT infusion should be stopped if manganese levels rise into the potentially toxic range (please refer to appropriate reference ranges). Particular attention must be paid when the product is given to patients with reduced biliary excretion, since it could interfere with the biliary elimination of manganese, copper and zinc, leading to accumulation and overdose. NUTRYELT should be used with caution in patients with impaired renal function as excretion of some trace elements (selenium, fluoride, chromium, molybdenum and zinc) may be significantly decreased. In patients with renal, hepatic impairments or mild cholestasis the posology should be adapted. In patients undergoing medium to long-term parenteral nutrition, there is an increased occurrence of iron, zinc and selenium deficiency. In such circumstances, when necessary, the dosage should be adapted with the use of an extra supply of solutions, which contain only these individual components. For patients receiving repeated blood transfusions, a risk of iron overload can occur. Parenterally administered iron preparations can cause hypersensitivity reactions including serious and potentially fatal anaphylactic/anaphylactoid reactions. The risk is enhanced for patients with known allergies including drug allergies
Interaction with other medicinal products and other forms of interaction:
Combinations not recommended:
+ Iron salts (oral route):
Fainting or shock attributed to the rapid release of iron from its complex shape and transferrin saturation.
Fertility, pregnancy and lactation
Pregnancy
No safety data for NUTRYELT are available when it is administered during pregnancy and lactation. Therefore, NUTRYELT should not be used during pregnancy and lactation except after special consideration and if it is absolutely necessary.
Undesirable effects: Pain at the application site, cases of hypersensitivity reactions including fatal anaphylactic reactions have been reported in patients receiving IV iron-containing products.
Overdose: If overdose is suspected, treatment with NUTRYELT should be withdrawn. Overdose should be confirmed by appropriate laboratory tests.
PHARMACEUTICAL PARTICULARS:
Incompatibilities
- NUTRYELT must not be used as a vehicle for other drugs.
- NUTRYELT, as with other trace element solutions, cannot be added directly
to inorganic phosphate (additive) solutions.
- Degradation of ascorbic acid in parenteral nutrition mix is accelerated by
trace elements
Shelf life
3 years. After dilution, chemical and physical in-use stability has been demonstrated for 48 h at 25°C protected from light. From a microbiological point of view, the product should be used immediately after dilution. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user.
Date of preparation: March 2017