Finomel
Finomel gives you more fish oil than any other three-chamber bag available today.1-8

Finomel is Baxter’s three-chamber bag (3CB) for clinicians who prefer to provide a lipid containing fish oil to their adult patients on parenteral nutrition (PN) therapy. Finomel contains 20% omega-3 rich fish oil and delivers the fish oil dose recommended by international guidelines.1,2,9 Finomel is available in a range of bag sizes and formulations for both central and peripheral administration to meet the unique nutritional needs of PN patients.1,2
See full prescribing information for Finomel
See full prescribing information for Finomel Perifer

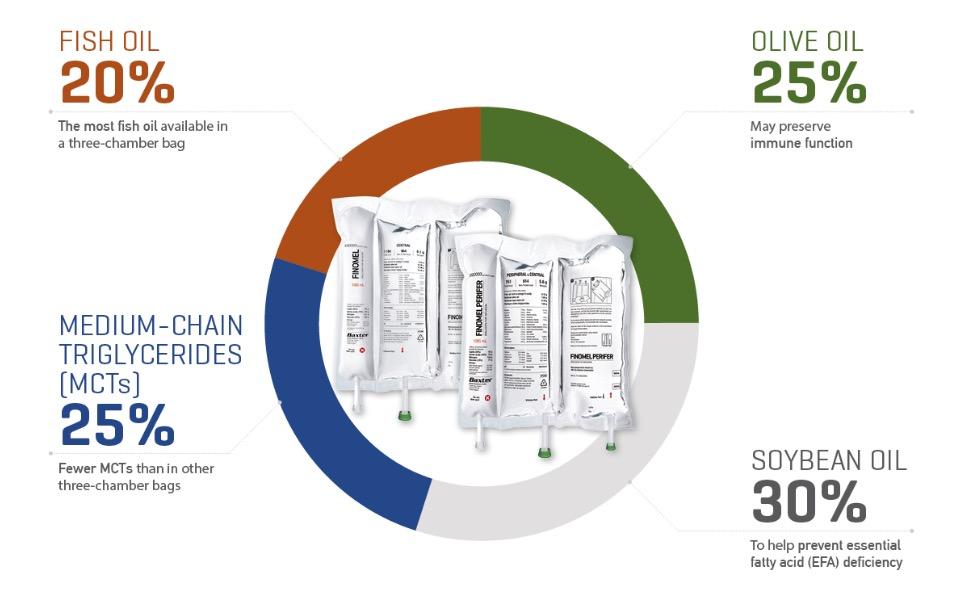
More fish oil than other 3CBs
With 7.6 g/L of fish oil rich in omega-3, Finomel contains 33% more fish oil than any other 3CB.1-8

Recommended fish oil dose in less fluid
With the highest concentration of fish oil, Finomel delivers the ESPEN guidelines recommended fish oil dose of 0.1-0.2 g/kg/day in less fluid compared to alternative solutions.1-9
A balanced four-oil lipid profile

Finomel
Download the Finomel and Finomel Perifer Compatibility Guide for compatibility and stability information
Learn more about Clinical Nutrition

Related Products
Important safety information
This abbreviated summary of product characteristics (SPC) is intended for international use. Please note that it may differ from the licensed SPC in the country where you are practicing. Therefore, please always consult your country-specific SPC or package leaflet.
NAME OF THE MEDICINAL PRODUCT: FINOMEL, emulsion for infusion, FINOMEL PERIFER, emulsion for infusion
COMPOSITION
For FINOMEL: 3-compartment plastic bag containing a sterile non-pyrogenic combination of a 42% glucose solution, a 10% amino acid solution with electrolytes, and a 20% lipid emulsion.
For FINOMEL PERIFER: 3-compartment plastic bag containing a sterile non-pyrogenic combination of a 13% glucose solution, a 10% amino acid solution with electrolytes, and a 20% lipid emulsion.
For FINOMEL/FINOMEL PERIFER: Active substances: Fish oil-rich in omega-3-acids; Olive oil refined; soybean oil refined, Medium chain triglycerids; Alanine; Arginine; Glycine; Histidine; Isoleucine; Leucine; Lysine; Methionine; Phenylalanine; Proline; Serine; Threonine; Tryptophan; Tyrosine; Valine; Sodium acetate, trihydrate; Sodium glycerophosphate, hydrated; Potassium chloride; Magnesium sulfate, heptahydrate; Calcium chloride, dihydrate; Zinc sulfate heptahydrate; Glucose monohydrate. Check full SPC for full quali/quantitative composition.
Therapeutic indications
Finomel/Finomel Perifer is indicated for parenteral nutrition in adult patients when oral or enteral nutrition is impossible, insufficient or contraindicated.
Method of Administration
Finomel: Intravenous use, infusion into a central vein. Finomel Perifer: Intravenous use, infusion intro a peripheral or central vein.
Contraindications
Hypersensitivity to fish-, egg-, soya- peanut- proteins, corn/corn product or to any of the active substances or excipients; Severe hyperlipidemia; Severe hepatic impairment; Severe blood coagulation disorders; Congenital abnormalities of amino acid metabolism; Severe renal impairment without access to hemofiltration or dialysis; Uncontrolled hyperglycemia; Pathologically elevated serum levels of any of the included electrolytes; General contraindications to infusion therapy: acute pulmonary oedema, hyperhydration, and decompensated cardiac insufficiency; Unstable conditions (e.g. severe post-traumatic conditions, uncompensated diabetes mellitus, acute myocardial infarction, stroke, embolism, metabolic acidosis, severe sepsis, hypotonic dehydration and hyperosmolar coma).
Undesirable effects
The following adverse reactions have been reported with other similar products. The frequency of these events cannot be estimated from available data:
| System Organ Class (SOC) | Preferred MedDRA term |
|---|---|
| Immune system disorders | Hypersensitivity |
| Metabolism and nutrition disorders | Refeeding syndrome, Hyperglycemia |
| Nervous system disorders | Dizziness, Headache |
| Vascular disorders | Thrombophlebitis |
| Respiratory, thoracic and mediastinal disorders | Pulmonary embolism |
| Respiratory distress | Dyspnea |
| Gastrointestinal disorders | Nausea, Vomiting |
|
General disorders and administration site conditions |
Pyrexia, Extravasation |
| Investigations | Hepatic enzyme increased |
| Injury, poisoning and procedural complications | Fat overload syndrome, Parenteral nutrition associated liver disease |
For a detailed composition, posology, Special warnings and precautions, incompatibilities, interactions, pharmacological properties and pharmaceutical particulars, please refer to the full SPC. Medicinal products are subject to medical prescription. Revision date: Nov 2018