Junyelt
A balanced multi-trace element solution containing five essential trace elements for neonatal and pediatric patients that helps avoid micronutrient deficiencies and aligns with international guidelines.
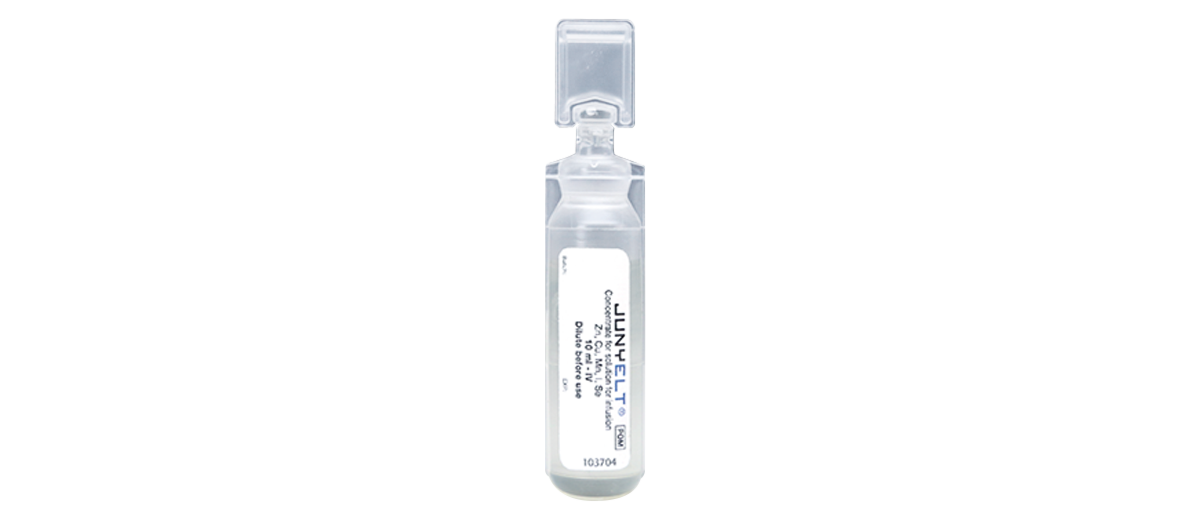
Micronutrients are a key component of parenteral nutrition (PN) and essential for optimal growth and development. Junyelt provides a concentrated solution of five essential trace elements: zinc, manganese, copper, selenium, and iodine, and is formulated to meet the basal requirements for these micronutrients. Junyelt can be used with macronutrients as part of the nutritional therapy of preterm and term newborns, infants, and children.1
Junyelt and pediatric patient care. Made for each other

Compatible
Compatibility data exists for admixture with Numeta, Primene, ClinOleic, and compounded solutions.9

Less manganese
Junyelt contains less manganese than other pediatric trace element solutions.1, 5-7 Excess manganese can accumulate in the brain and cause neurotoxicity and can be toxic in the presence of chlolestasis.3, 8
Easy to use
Luer-fit ampoule compatible with Luer-slip and Luer-lock syringes: designed to avoid needlestick injuries.10
Aligned with international guidelines
A daily dose of Junyelt covers the basal requirements of zinc, manganese, copper, selenium, and iodine in preterm and term newborns, infants and children.1,4
Junyelt
Download the Baxter Parenteral Nutrition Compatibility Guide for compounding and multi-chamber bag compatibility and stability information.
Learn more about Clinical Nutrition

Related Products
This abbreviated summary of product characteristics (SPC) is intended for international use. Please note that it may differ from the licensed SPC in the country where you are practicing. Therefore, please always consult your country-specific SPC or package leaflet.
JUNYELT, concentrate for solution for infusion
QUALITATIVE AND QUANTITATIVE COMPOSITION
One ampoule (10 ml) contains: Zinc gluconate 6970 µg; Copper gluconate 1428 µg, Manganese gluconate 40.52 µg, Potassium iodide 13.08 µg, Sodium selenite 43.81 µg. Excipients: hydrochloric acid, water for injections.
CLINICAL PARTICULARS
Therapeutic indications:
JUNYELT is used as part of the intravenous nutrition of preterm and term newborns, infants and children. It is intended to meet the basal requirements for trace elements.
Posology and method of administration:
Preterm, and term newborns, infants and children (weighing 20 kg or less): Basal requirements of the included trace elements are covered by 1 ml of JUNYELT per kg body weight per day to a maximum daily dose of 20 ml. Children (weighing more than 20 kg): A daily dose of 20 ml JUNYELT should meet basal trace element requirements. JUNYELT should be supplemented with a single zinc injectable solution in case of administration to preterm infants to reach a total zinc parenteral intake of 450-500 µg/kg/day. A daily iron infusion is recommended when preterm infants are receiving long term parenteral nutrition (> 3 weeks), and molybdenum add-on in case of parenteral nutrition > 4 weeks.
Method of administration:
Intravenous route: JUNYELT is not intended to be administered in its current presentation. It should be diluted according to the final desired osmolarity.
Contraindications:
Patients with known hypersensitivity to one of the actives substances and to the excipients. In case of Wilson’s disease and if serum concentrations of any of the trace elements contained in JUNYELT are elevated.
Special warnings and precautions for use:
The solution should be used after an accurate control of the patient clinical and biological parameters. In paediatrics, individual trace element requirements may vary based on factors such as age, weight, underlying disease state and duration of parenteral nutrition. Blood manganese levels should be regularly monitored in case of prolonged artificial nutrition. A dose reduction may be necessary or infusion of JUNYELT should be stopped if manganese levels rise into the potentially toxic range. The occurrence of neurological signs must evoke the possibility of a manganese overdose. Particular attention must be paid when the product is given to patients with reduced biliary excretion, since this could interfere with the biliary elimination of manganese, copper and zinc, leading to accumulation and overdose. Copper overdose must be considered in the presence of nausea, vomiting, gastralgia. In patients with hepatic impairments or mild cholestasis the posology should be adapted. Besides, in case of pronounced cholestasis blood copper levels and hepatobiliary parameters should be monitored. JUNYELT should be used with caution in patients with impaired renal function, as excretion of some trace elements (selenium and zinc) may be significantly decreased, leading to accumulation and overdose. In patients with renal impairments, the posology should be adapted. JUNYELT should be used with caution in patients with manifest hyperthyroidism. In patients undergoing medium to long-term parenteral nutrition, there is an increased frequency of copper, zinc and selenium deficiency. In such circumstances, when necessary, the dosage should be adapted with the use of an extra supply of solutions, which contain only these individual components. Because of a risk of precipitation, drugs or electrolytes should not be added to JUNYELT before the later has been diluted. The compatibility profile of infusion solutions administered through the same line should be verified. No adjustment of JUNYELT is required in case of additional intake of iodine through iodine-based antiseptic. This product contains 11.6 µg of sodium per ampoule, i.e. essentially “sodium free” and 3.1 µg of potassium per ampoule, i.e. essentially “potassium free”.
Interaction with other medicinal products and other forms of interaction: No interaction studies have been performed.Fertility, pregnancy and lactation:Not relevant.Undesirable effects: Pain at the application site.
Overdose: If overdose is suspected, treatment with JUNYELT should be withdrawn. Overdose should be confirmed by appropriate laboratory tests.
PHARMACEUTICAL PARTICULARS:
Incompatibilities: JUNYELT must not be used as a vehicle for other drugs.This medicinal product must not be mixed with other medicinal products except those mentioned in section 6.6 of SPC.
Shelf life: 36 months. After dilution, chemical and physical in-use stability has been demonstrated for 48 h at 25°C. From a microbiological point of view, the product should be used immediately after dilution. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user.
Date of preparation: April 2020