Important safety information
This abbreviated summary of product characteristics (SPC) is intended for international use. Please note that it may differ from the licensed SPC in the country where you are practicing. Therefore, please always consult your country-specific SPC or package leaflet.
NAME OF THE MEDICINAL PRODUCT
Numeta G13E emulsion for infusion
Numeta G16E emulsion for infusion
Numeta G19E emulsion for infusion
QUALITATIVE AND QUANTITATIVE COMPOSITION
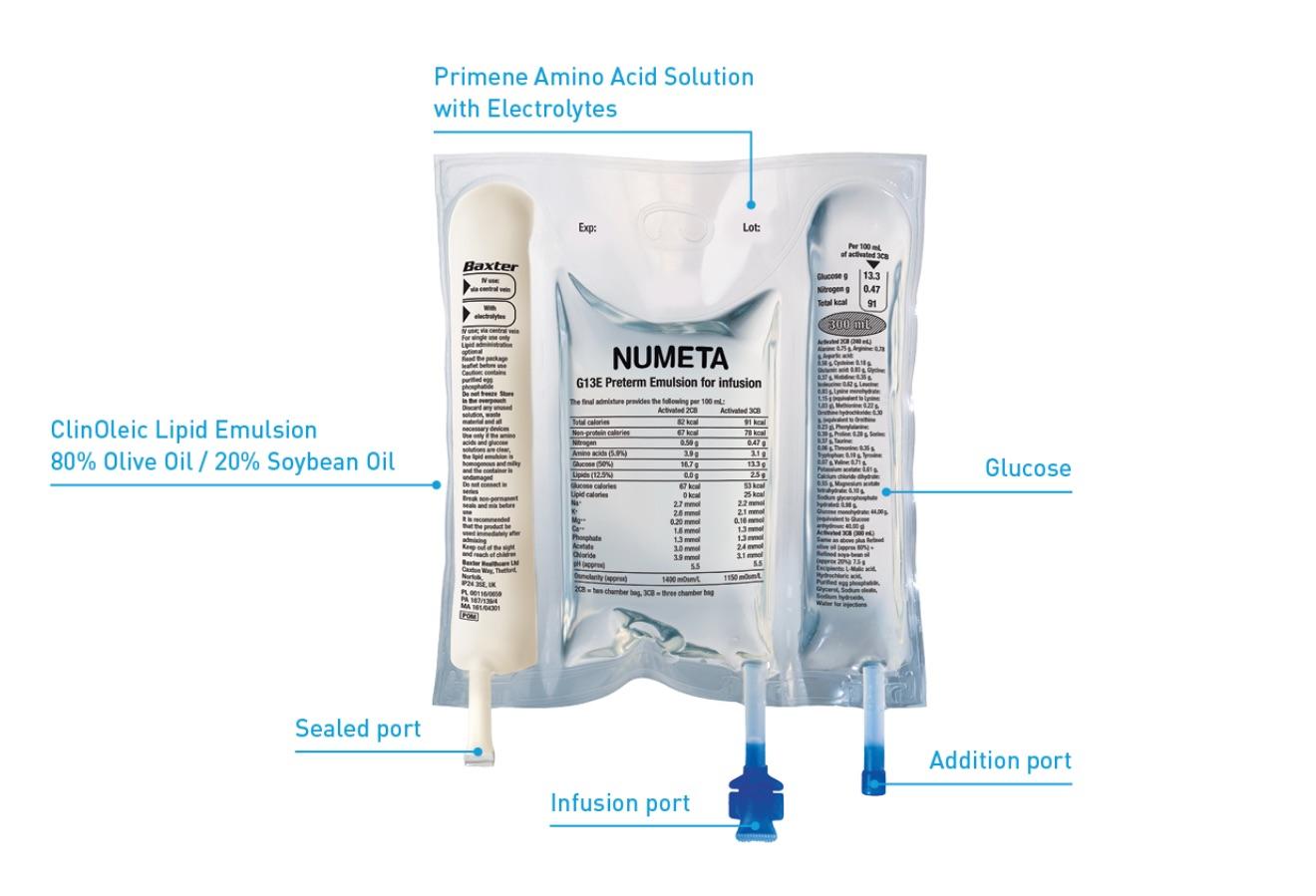
This medicinal product is presented in the form of a three-chamber-bag. Each bag contains a sterile non-pyrogenic combination of a glucose solution, a pediatric amino acids solution, with electrolytes, and a lipid emulsion, as described below
|
Product
|
Container Size
|
50% glucose solution
|
5.9% amino acids solution with electrolytes
|
12.5% lipid emulsion
|
|
Numeta G13E
|
300 mL
|
80 mL
|
160 mL
|
60 mL
|
|
Numeta G16E
|
500 mL
|
155 mL
|
221 mL
|
124 mL
|
|
Numeta G19E
|
1000 mL
|
383mL
|
392 mL
|
225 mL
|
If lipid administration is undesirable, the design of the bag makes it possible to activate only the peel seal between the amino acids/electrolytes and glucose chambers, leaving the peel seal between the amino acids and lipid chambers intact. The content of the bag can subsequently be infused with or without lipids.
CLINICAL PARTICULARS
Therapeutic indications
Numeta G13E is indicated for parenteral nutrition in preterm newborn infants when oral or enteral nutrition is not possible, insufficient or contraindicated.
Numeta G16E is indicated for parenteral nutrition in term newborn infants and children up to 2 years when oral or enteral nutrition is not possible, insufficient or contraindicated.
Numeta G19E is indicated for parenteral nutrition in children older than 2 years and adolescents 16-18 years old when oral or enteral nutrition is not possible, insufficient or contraindicated.
Posology and method of administration
Posology
The dosage depends on energy expenditure, the patient’s weight, age, clinical status, and on the ability to metabolize the constituents of Numeta, as well as on additional energy or proteins given orally/enterally. Total electrolyte and macronutrient composition is dependent on the number of activated chambers.
The maximum daily dose should not be exceeded. Due to the static composition of the multi-chamber bag, the ability to simultaneously meet all nutrient needs of the patient may not be possible. Clinical situations may exist where patients require amounts of nutrients varying from the static composition.
The maximal recommended hourly rate of infusion and volume per day depends on the constituent. The first of these limits to be reached sets the maximum daily dose. The guidelines for maximum recommended hourly rate of infusion and volume per day are:
For Numeta G13E
|
|
Activated 2CB (240 mL)
|
Activated 3CB (300 mL)
|
|
Maximal rate of infusion in mL/kg/h
|
5.1
|
6.4
|
|
Maximal amount in mL/kg/day
|
102.3
|
127.9
|
For Numeta G16E
|
|
Activated 2CB (376 mL)
|
Activated 3CB (500 mL)
|
|
Maximal rate of infusion in mL/kg/h
|
5.8
|
5.5
|
For Numeta G19E
|
|
Activated 2CB (775 mL)
|
Activated 3CB (1000 mL)
|
|
Maximal rate of infusion in mL/kg/h
|
4.7
|
4.6
|
|
Maximal amount in mL/kg/day
|
64.8
|
83.6
|
Method of administration
Numeta G13E and G16E: When used in neonates and children below 2 years the solution (in bags and administration sets) should be protected from light exposure until administration is completed.
Numeta G19E: The solution (in bags and administration sets) should be protected from light exposure from point of admixture through administration.
The flow rate should be increased gradually during the first hour. Upon discontinuation of Numeta, the flow rate should be decreased gradually during the last hour. The administration flow rate must be adjusted taking into account the dose being administered, the daily volume intake, and the duration of the infusion.
In preterm newborn infants, continuous parenteral administration over 24 hours is usually recommended; however, the same bag should not be activated, hung and infused longer than 24 hours. Cyclic infusions should be managed according to the patient’s metabolic tolerance.
Treatment with parenteral nutrition may be continued for as long as is required by the patient’s clinical conditions.
For Numeta G13E / Numeta G16E / Numeta G19E
Due to its high osmolarity, undiluted Numeta can only be administered through a central vein. However, sufficient dilution of Numeta with water for injection lowers the osmolarity and allows peripheral infusion.
Contraindications
The general contraindications for administering Numeta as an activated two-chamber-bag for intravenous infusion are as follows:
- Hypersensitivity to egg, soy or peanut proteins, or to any of the active substances, excipients, or components of the container
- Congenital abnormality of the amino acid metabolism
- Pathologically elevated plasma concentrations of sodium, potassium, magnesium, calcium and/or phosphorus
- Concomitant treatment with ceftriaxone even if separate infusion lines are used
- Severe hyperglycaemia
The addition of lipids (administering Numeta as an activated three-chamber-bag for intravenous emulsion) is contraindicated in the following additional clinical situations:
- Severe hyperlipidaemia, or severe disorders of lipid metabolism characterized by hypertriglyceridaemia.
Undesirable effects
The pooled data from clinical trials and the postmarketing experience indicate the following adverse drug reactions (ADRs) related to Numeta:
Hypertriglyceridaemia a
|
Clinical Trial and Post-marketing experience Adverse Reactions
|
|
System Organ Class (SOC)
|
Preferred MedDRA Term
|
Frequencyb
|
|
METABOLISM AND NUTRITION DISORDERS
|
Hypophosphataemia a
|
Common
|
| |
Hyperglycaemia a
|
Common
|
| |
Hypercalcaemia a
|
Common
|
| |
Hypertriglyceridamia a |
Common
|
| |
Hyperlipidaemia a
|
Uncommon
|
| |
Hyponatraemia a
|
Common
|
|
HEPATOBILIARY DISORDERS
|
Cholestasis
|
Uncommon
|
|
SKIN AND SUBCUTANEOUS TISSUE DISORDERS
|
Skin necrosis c
|
Not known
|
| |
Soft tissue injury c
|
Not known
|
|
GENERAL DISORDERS AND ADMINISTRATION SITE CONDITION
|
Extravasation c
|
Not known
|
a Blood samples drawn during the infusion (without fasting conditions).
b Frequency is based upon the following categories: Very Common (≥1/10); Common (≥1/100 - <1/10), Uncommon (≥1/1,000 - <1/100), Rare (≥1/10,000 - <1/1,000), Very Rare (<1/10,000), Not known (cannot be estimated based on available data).
c These adverse reactions have been reported only for Numeta G13E and G16E when peripherally administered with insufficient dilution
The following adverse reactions have been reported with other parenteral nutrition admixtures:
Fat overload syndrome: reduced ability to remove the lipids contained in Numeta may result in a “fat overload syndrome” which may be caused by overdose and/or infusion rate higher than recommended, and is associated with a sudden deterioration in the patient’s clinical condition. It is characterized by hyperlipidemia, fever, liver fatty infiltration, hepatomegaly (deteriorating liver function), anemia, leukopenia, thrombocytopenia, coagulation disorders and central nervous system manifestations (e.g.coma). The syndrome is usually reversible when the infusion of the lipid emulsion is stopped.
Pulmonary vascular precipitates (pulmonary vascular embolism and respiratory distress).
Precautions
For Numeta G13E, Numeta G16E and Numeta G19E:
Do not add other medicinal products or substances to one of the three chambers of the bag or to the reconstituted solution/ emulsion without first confirming their compatibility and the stability of the resulting preparation.
Cardiovascular: Use with caution in patients with pulmonary edema or heart failure. Fluid status should be closely monitored. Renal: Use with caution in patients with renal insufficiency. Fluid and electrolyte status, including magnesium, should be closely monitored in these patients. Severe water and electrolyte equilibration disorders, severe fluid overload states, and severe metabolic disorders should be corrected before starting the infusion. Hepatic/Gastrointestinal: Use with caution in patients with severe liver insufficiency, including cholestasis, or elevated liver enzymes. Liver function parameters should be closely monitored. Endocrine and Metabolism: Metabolic complications may occur if the nutrient intake is not adapted to the patient’s requirements, or the metabolic capacity of any given dietary component is not accurately assessed. Adverse metabolic effects may arise from administration of inadequate or excessive nutrients or from inappropriate composition of an admixture for a particular patient’s needs. Hematologic: Use with caution in patients with severe blood coagulation disorders. Blood count and coagulation parameters should be closely monitored.
For Numeta G13E and Numeta G16E: Light exposure of solutions for intravenous parenteral nutrition, especially after admixture with trace elements and/or vitamins may have adverse effects on clinical outcome in neonates, due to generation of peroxides and other degradation products. When used in neonates and children below 2 years, Numeta G13E and Numeta G16E should be protected from ambient light until administration is completed.
Numeta G19 should be protected from light from the point of admixture through administration.
For Numeta G16E
Hypermagnesaemia: Numeta G16E provides 0.3 mmol/kg/d of magnesium when administered at maximum dose There is a possibility that this may lead to hypermagnesaemia. If serum magnesium levels are elevated (above reference range normal values) the infusion of Numeta G16E should be stopped or infusion rate reduced as deemed clinically appropriate and safe.
For the detailed posology, Special warnings and precautions for use, interactions, pharmacological properties and pharmaceutical particulars, please refer to the full SPC. Medicinal products are subject to medical prescription Revised August 2020
This abbreviated summary of product characteristics (SPC) is intended for international use. Please note that it may differ from the licensed SPC in the country where you are practicing.
Therefore, please always consult your country-specific SPC or package leaflet.
NAME OF THE MEDICINAL PRODUCT
PRIMENE 10%
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each litre of the infusion solution contains:
L-Isoleucine 6.70g ; L-Leucine 10.00g ; L-Valine 7.60g ; L-Lysine 11.00g ; L-Methionine 2.40g ; L-Phenylalanine 4.20g ; L-Threonine 3.70g ; L-Tryptophan 2.00g ; L-Arginine 8.40g ; L-Histidine 3.80g ; L-Alanine 8.00g ; L-Aspartic Acid 6.00g ; L-Cysteine 1.89g; L-Glutamic Acid 10.00g ; Glycine 4.00g ; L-Proline 3.00g ; L-Serine 4.00g ;L-Tyrosine 0.45g ; L-Ornithine Hydrochloride 3.18g ; Taurine 0.60g
In a formulation also containing L-Malic acid, as described in the application.
CLINICAL PARTICULARS
Therapeutic Indications
Primene 10% is indicated in 1) children and infants 2) neonates, at term or premature, of normal or low birth weight when oral or enteral nutrition is impossible, insufficient or contraindicated.
Posology and Method of Administration
Dosage depends on the age, weight and protein catabolism of the child:
The usual range is: 15 – 35 ml of Primene 10%/kg/24 hours
The infusion rate should not exceed 0.05 ml/kg/min.
Recommended flow rates: Neonates and Infants: continuous infusion (over 24 hours).
Children: continuous infusion (over 24 hours) or cyclic infusion (over about 12 hours in 24).
The flow rate should be adjusted according to the dosage, the characteristics of the infusion solution, the total volume intake per 24 hours and the infusion duration.
Route of administration: Primene 10% alone should be administered in a central vein.
Primene 10% in co-administration or as a mixture should be administered according to the final osmolarity of the solution infused, in a peripheral or central vein.
Mode of administration: Primene 10% is usually administered with a source of energy appropriate for the needs of the child, either by co-administration or as a mixture.
Primene 10% may be included in the composition of nutritive mixtures combining carbohydrates, lipids, electrolytes, trace elements and vitamins to meet nutrient needs and prevent deficiencies and complications from developing, when compatibility and stability are known.
Contraindications
- Hypersensitivity to any of the active substances or to any of the excipients
- Congenital abnormality in the metabolism of one or more amino-acids.
Special Warnings and Special Precautions for Use
Primene 10% must be used with caution where severe restriction of water intake is necessary, e.g. cardiac, respiratory or renal failure. In cases of renal insufficiency, the nitrogen intake must be adjusted according to the Childs renal clearance. Caution should be exercised in cases of hepatic insufficiency with careful monitoring of blood ammonia levels. Careful monitoring of the infusion and of the clinical and biochemical conditions of the child is essential. In view of its osmolality, Primene 10% should not be infused alone into a peripheral vein. When used in neonates and children below 2 years of age, Primene should be protected from ambient light until administration is completed.
Undesirable effects
From post marketing reports: Immune system disorders: Hypersensitiviy reaction manifested by face oedema, eyelid oedema, rash- frequency not known.
Adverse reactions reported with parenteral amino acid products include: azotaemia, hyperammonaemia, anaphylactic/anaphylactoid reactions, hepatic failure, raised blood urea nitrogen in children with renal insufficiency, metabolic acidosis, pulmonary vascular precipitates, necrosis at the infusion site, infusion site thrombophlebitis, etc.
For a detailed posology, Special warnings and precautions, incompatibilities, interactions, pharmacological properties and pharmaceutical particulars, please refer to the full SPC. Medicinal products are subject to medical prescription
Jan 2020
Important safety information
This abbreviated summary of product characteristics (SPC) is intended for international use. Please note that it may differ from the licensed SPC in the country where you are practicing.
Therefore, please always consult your country-specific SPC or package leaflet.
ClinOleic 20%:
Emulsion for infusion.
QUALITATIVE AND QUANTITATIVE COMPOSITION
Per 100 ml: Refined olive oil and refined soybean oil* 20.00 g corresponding to a content of essential fatty acids 4.00 g. Energy content 2000 kcal/l (8.36 MJ/l).Lipid content (olive and soybean oil) 200 g/l.Osmolarity 270 mOsm/l.pH 6 -8 Density 0.986.Phospholipids provide 47 milligrams or 1.5 mmol of phosphorus per 100 ml.
*Mixture of refined olive oil (approximately 80%) and refined soybean oil (approximately 20%).
CLINICAL PARTICULARS
Therapeutic indications:
Indicated as a source of lipids for patients requiring parenteral nutrition, when oral or enteral nutrition is impossible, insufficient or contraindicated.
Posology and method of administration:
ClinOleic 20% contains 200 mg/ml of lipids.
The posology depends on energy expenditure, the patient’s clinical status, body weight, and ability to metabolize ClinOleic 20%, as well as additional energy given orally/enterally. Therefore, the dosage should be individualized and the bag size chosen accordingly.
Dosage:
In adults: The posology is 1 to a maximum of 2 g lipids/kg/day. The initial infusion rate must be slow and not exceed 0.1 g lipids or 0.5 ml (10 drops) per minute for 10 minutes then gradually increased until reaching the required rate after half an hour. Never exceed 0.15 g lipids/kg/hour (0.75 ml/kg/hour). Adults per kg of body weight: Usual lipid dosage: 1 to 2 g/kg/day. Infused volume of ClinOleic 20%:5 to 10 ml/kg/day. Adults for 70 kg: Usual lipid dosage: 70 to 140 g/day. Infused volume of ClinOleic 20%: 350 to 700 ml/day.
In children: ClinOleic 20% should be administered as a continuous 24h/day infusion. It is recommended not to exceed a daily dose of 3g-lipids/kg b.w. and an infusion rate of 0.15 g lipids/kg b.w./h. Daily dose should be increased gradually during the first week of administration.
In premature newborns and low birth weight infants: The use of ClinOleic 20% is restricted to premature infants of 28 weeks of gestational age or more. ClinOleic 20% should be administered as a continuous 24h/day infusion. The initial daily dose should be 0.5-1.0g lipids/kg b.w. The dose may be increased by 0.5-1.0g lipids/kg b.w. every 24 hours up to a daily dose of 2.0 g lipids/kg b.w. Usage in nutritive admixtures (with glucose and amino acids): “Breaking” or “oiling out” of the emulsion can be visibly identified by accumulation of yellowish droplets or particles in the admixture.
Contraindications:
- hypersensitivity to the active substance or to any of the excipients (e.g.: egg or soybean protein)
- severe dyslipidemia and non-corrected metabolism disorders including lactic acidosis and uncompensated diabetes
Special warnings and special precautions for use:
The infusion must be stopped immediately if any abnormal signs or symptoms of an allergic reaction (such as sweating, fever, shivering, headache, skin rashes or dyspnoea) develop. This medicinal product contains soybean oil and egg phospholipids. Soybean and egg proteins may cause hypersensitivity reactions. Cross-allergic reactions between soybean and peanut proteins have been observed.
During short-term or long-term intravenous nutrition, alkaline phosphatases and total bilirubin should be checked at regular intervals, depending on the health status of the patient. The blood sugar, serum triglycerides, the acid-base balance, electrolytes, serum osmolarity, kidney function, coagulation parameters and the blood count must be checked at regular intervals.
Parenteral nutrition should be used with caution in patients with pre-existing liver disease or liver insufficiency. Liver function parameters should be closely monitored in these patients.
Parenteral Associated Liver Diseases (PNALD) including cholestasis, hepatic staetosis, fibrosis and cirrhosis, possibly leading to hepatic failure, as well as cholecystitis and cholelithiasis are known to develop in some patients on parenteral nutrition. The etiology of these disorders is thought to be multifactorial and may differ between patients. Patients developing abnormal laboratory parameters or other signs of hepatobiliary disorders should be assessed early by a clinician knowledgeable in liver diseases in order to identify possible causative and contributory factors, and possible therapeutic and prophylactic interventions.
ClinOleic 20% should be administered with caution in case of neonatal hyperbilirubinemia (total serum bilirubin > 200 μmol/ l). Total bilirubin levels should be monitored closely. As other lipid emulsions, ClinOleic 20% should be used in extremely premature and/or very low birth-weight infant under the close supervision of a neonatologist. There is clinical experience for ClinOleic 20% infusion time, up to 7 days in neonates and up to 2 months in children. Light exposure of solutions for intravenous parenteral nutrition, especially after admixture with trace elements and/or vitamins, may have adverse effects on clinical outcome in neonates, due to generation of peroxides and other degradation products. When used in neonates and children below 2 years, Clinoleic should be protected from ambient light until administration is completed
Interactions with other medicaments and other forms of interaction: Complete information about incompatibilities is not available. ClinOleic 20% contains vitamin K, naturally present in lipid emulsions. The amount of Vitamin K in recommended doses of ClinOleic 20%, is not expected to influence effects of coumarin derivatives.
The lipids contained in this emulsion may interfere with the results of certain laboratory tests if the blood sample is taken before the lipids are eliminated from the serum (these are generally eliminated after a period of 5 to 6 hours without receiving lipids). Refer to the laboratory testing system product information regarding potential assay interference associated with lipemic samples.
Pregnancy and lactation: The safety of administration of ClinOleic 20% during pregnancy and lactation has not been established. Therefore, ClinOleic 20% should not be used during pregnancy and lactation except after special consideration.
Undesirable effects:
Common: nausea vomiting, hyperglycaemia, mean arterial pressure decreased.
Uncommon: Dyspnea, Cholestasis, Blood bilirubin increased, Hepatic enzyme increased.
Overdose: A reduced ability to remove the lipids may result in a “fat overload syndrome” which may be caused by overdose, the effects of which are usually reversible after the lipid infusion is stopped.
PHARMACEUTICAL PARTICULARS:
List of excipients:
Egg phospholipids, glycerol, sodium oleate, sodium hydroxide, water for injections.
Incompatibilities:
Complete information about incompatibilities is not available. Never add medication or electrolytes directly to the lipid emulsion. If it is necessary to introduce additives, verify the compatibility and mix thoroughly before administration to the patient.
Shelf life:
18 months in plastic bag in its overwrap. When used in neonates and children below 2 years, the solution (in bags and administration sets) should be protected from light exposure until administration is completed.
Special precautions for storage:
Do not store above 25°C.Do not freeze. Keep the container in the outer carton.
Medicinal products subject to medical prescription.
Date of preparation: Mar 2020
Numeta G13E emulsion for infusion ENG SmPC
Numeta G16E emulsion for infusion ENG SmPC
Numeta G19E emulsion for infusion ENG SmPC
ClinOleic SmPC
Primene SmPC